CLP Regulation No. 1272/2008 What does this new decision mean for you? How do you remain compliant now?
The CLP Regulation (EC 1272/2008) defines how chemical substances and mixtures are classified, labeled, and packaged in the EU. This ensures uniform hazard communication and enhances safety for users and the environment.
History of Classification, Labelling, and Packaging:
Before the introduction of CLP, the DSD (Dangerous Substances Directive) and DPD (Dangerous Preparations Directive) were in place, each with its own symbols and regulations. With the introduction of the Globally Harmonized System (GHS) by the United Nations (UN) in 2003, efforts began towards a globally uniform approach. In 2009, CLP came into force in the EU, with a transition period until 2015, after which the old directives were completely phased out.
A revision of CLP
Since 2008, a lot has happened, and the regulations were due for change. The 2024 revision of the CLP-regulation improves chemical safety and information transparency.
Discover all the latest updates on CLP
- Online stores will need to clearly display the hazardous properties of products on their websites.
- Labeling becomes simpler through more flexible use of multilayer labels, the introduction of digital labels, and improved label readability.
- Advertisements and online offers must now include information about chemical hazards, so that consumers can make better-informed choices and a market for sustainable chemical consumer products can emerge.
- For the first time, clear rules are being introduced for the safe sale of household chemicals through refill stations, which contributes to less packaging and packaging waste. However, there are several restrictions for these refill stations. Not every product is allowed to be sold in such a refill station.
- In addition, a more user-friendly inventory of substances notified by the industry will be introduced.
- Explicit rules will be introduced for the classification of complex substances (substances with multiple components), with particular attention to natural complex substances, such as essential oils.
- Finally, poison control centers will receive more extensive information about medical emergencies, especially in cases of cross-border distribution
Hazard classes and hazard statements according to the CLP Regulation
A hazard class is a category within the CLP Regulation that indicates the type of hazard a chemical substance or mixture presents. This can include physical hazards (e.g., flammability), health hazards (e.g., carcinogenic), or environmental hazards (e.g., harmful to aquatic organisms).
Hazard statements (H-phrases) describe the specific risk of a substance within a given hazard class.
It is possible that a substance in your product, due to the CLP revision, belongs to a certain hazard class, and therefore new hazard statements need to be added to the label.



- The label
- The pictogram/pictograms
- The font

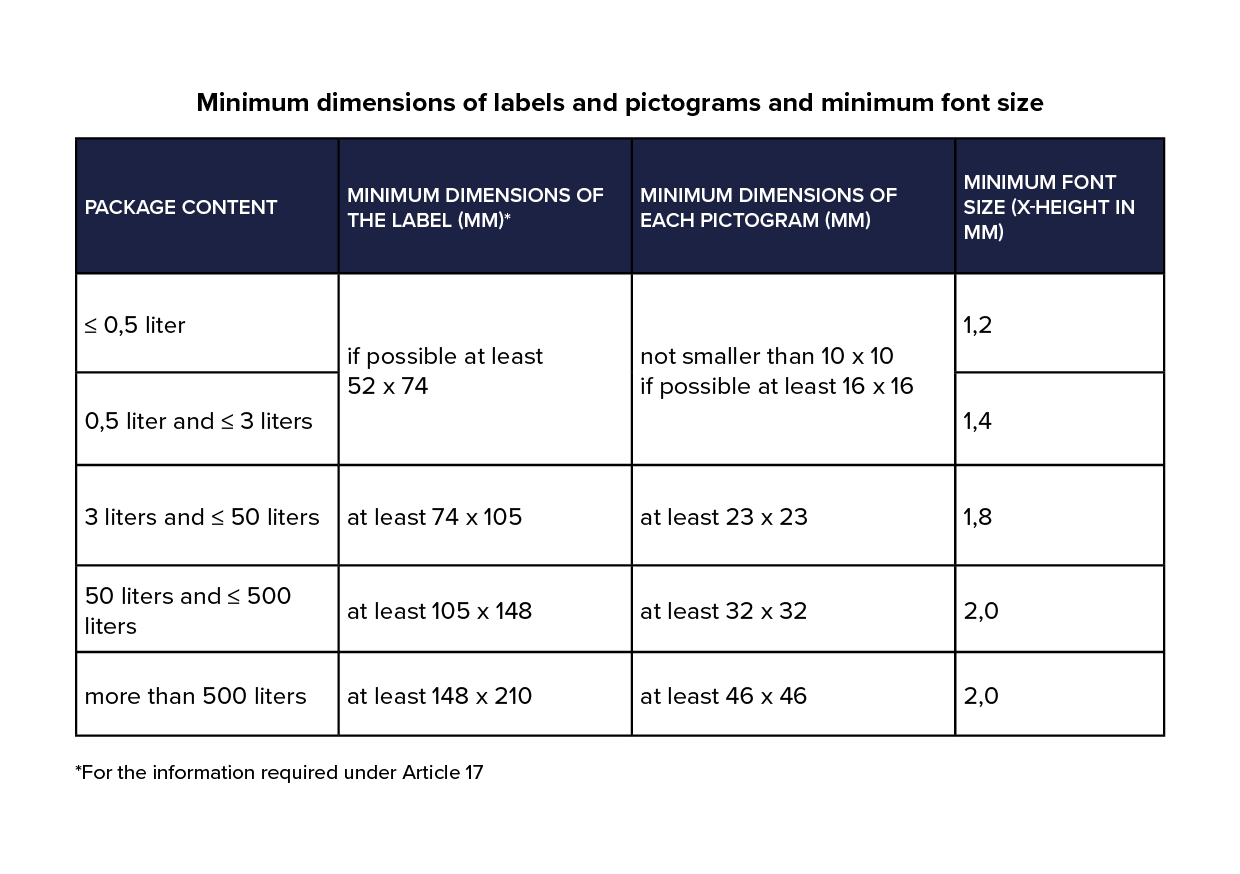

als lettertypegrootte 2mm is, dan is de interlinie 120% van 2mm = 2,4mm
Er wordt 1 zelfde lettertype gebruikt op het etiket

Fold-out label
A lot of products will require more information on the label, often in a larger font. Therefore, more space will clearly be needed.
Discover our multilayer label
Although the possibility of using multilayer labels already existed in the original CLP as a solution for small packaging, this "fold-out" label can now also be used as a standard.
There are some rules attached to this. For example, it is determined what should be placed on the front (red), inside (white), and on the base layer (yellow) of the multilayer label.
| Front (red) | Inside (white) | Base layer (yellow) | ||||
|
|
|
The digital label
This digital label has nothing to do with how the label is printed (digital vs flexo). The digital label in the CLP regulation revision refers to the possibility of making chemical hazard information digitally available as a supplement to the physical label.
This digital label is additional. It does not replace the physical label.
You could use a QR code that directs the consumer to a website. There, you could include, for example, the SDS (Safety Data Sheet) or a user manual.
You can also include the information that appears on the physical label, but under no circumstances should the information contradict or cause doubt about the validity of the information on the physical label.
If you use this digital label, an additional message must be included: "More hazard information is available online"
Regulations for digital labels:
- All required label information must be located in one place and clearly separated from other information.
- Searchability: Information must be easy to find.
- Accessibility: The digital label information must remain available for at least 10 years and be freely accessible.
- User-friendliness:
- No mandatory registration, passwords, or software installation.
- Accessibility for vulnerable groups must be ensured.
- Information must be accessible with a maximum of two clicks.
- Compatible with all common devices and browsers.
- Language choice must not depend on the user's geographical location.
- Privacy protection: It is prohibited to track or analyze user data for purposes other than what is absolutely necessary for providing digital labeling.
- Mandatory information that must appear on the physical label should be provided together in one place and separated from other information;
- Information on the digital label is searchable;
- The information on the digital label is accessible throughout the Union and remains available for at least ten years, or longer if required by Union legislation.
- The digital label is freely accessible, without registration, downloads, installation, or login.
Label update obligation
Although this already existed before, it was rather vaguely defined. The CLP revision now specifies this much more strictly:
- If a label requires new or stricter warnings, the supplier must make the adjustment as soon as possible and no later than within 6 months.
- For other label changes, a period of 18 months is provided.
- If the EU itself imposes a mandatory adjustment, the date set by the EU applies.
This will undoubtedly have an impact on inventory management.
Guidance on labelling (ECHA)
Later this year, the European Chemicals Agency (ECHA) will release a ‘guidance on labeling,’ but we would like to highlight some elements that are clearly defined.

